Good laboratory practice guidelines pdf
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
GOOD LABORATORY PRACTICES 1) Keep the manufacturer’s product insert for the laboratory test in use and be sure it is available to the testing personnel.
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
The document is a critical factor of the good laboratory Practice Documentation is the accepted method of recording information for future reference. The major documents that need to be provided are protocols, logbook for usage, maintenance and calibration of equipment there should be well established SOPs. The following are some of the information that is routinely recorded in a laboratory:
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
The Applicability of Good Laboratory Practice in Premarket Device Submissions: Questions and Answers – Draft Guidance for Industry and Food and Drug Administration Staff 08/23/13 10/16/18
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
Good Laboratory Practice (GLP) European Commission
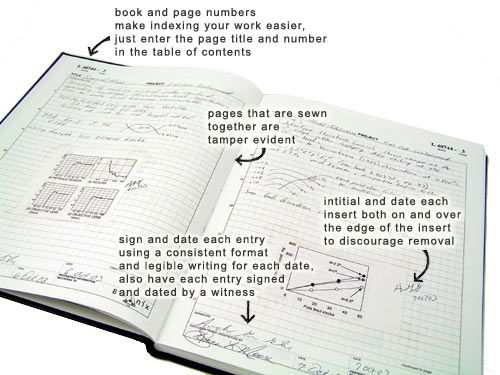
Revised guidelines for good practice in IVF laboratories
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
basic practices used in the laboratory. A manual is given to all newcomers; it includes all the A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices.
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015

Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
Good Laboratory Practice; Regulations and Guidelines; Regulatory Roadmap; Animal & Veterinary Product; D.I.G.I.T. Good Clinical Practice; Good Laboratory Practice . Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),

Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
This guideline was developed to help protect clinical trial participants and patients receiving marketed products from potential adverse effects of pharmaceuticals, while avoiding unnecessary use of animals and other resources.
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
Good Clinical Laboratory Practice Canary Ltd
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
—– GOOD LABORATORY PRACTICE STANDARDS INSPECTION MANUAL September 1993 Prepared by: Scientific Support Branch Laboratory Data Integrity Assurance Division Office of Compliance Monitoring (EN342W) Washington, DC 20460 (703) 308
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
This part prescribes good laboratory practices for conducting studies that support or are intended to support applications for research or marketing permits for
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. – good web application form design principles The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
MMWR Good Laboratory Practices for Waived Testing Sites
Good Laboratory Practices and the ISO 90012000 standards

ICMS
Good laboratory practice guidelines pdf” Keyword Found

[Code of Federal Regulations] National Toxicology Program
Good Laboratory Practice Standards Inspection Manual


Good Laboratory Practices Standards Compliance Monitoring
Tutorial Labcompliance
good food guide chefs hats 2017 – Good Laboratory Practice (QMS ISO 17025) 1 Day Training
GOOD LABORATORY PRACTICES Centers for Medicare


Good Laboratory Practice Standards Inspection Manual
[Code of Federal Regulations] National Toxicology Program
—– GOOD LABORATORY PRACTICE STANDARDS INSPECTION MANUAL September 1993 Prepared by: Scientific Support Branch Laboratory Data Integrity Assurance Division Office of Compliance Monitoring (EN342W) Washington, DC 20460 (703) 308
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
The Applicability of Good Laboratory Practice in Premarket
Good Laboratory Practices ppt SlideShare
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
The Applicability of Good Laboratory Practice in Premarket Device Submissions: Questions and Answers – Draft Guidance for Industry and Food and Drug Administration Staff 08/23/13 10/16/18
The document is a critical factor of the good laboratory Practice Documentation is the accepted method of recording information for future reference. The major documents that need to be provided are protocols, logbook for usage, maintenance and calibration of equipment there should be well established SOPs. The following are some of the information that is routinely recorded in a laboratory:
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
GOOD LABORATORY PRACTICES Centers for Medicare
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
basic practices used in the laboratory. A manual is given to all newcomers; it includes all the A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices.
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Good Laboratory Practice (GLP) European Commission
ICMS
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
The Applicability of Good Laboratory Practice in Premarket Device Submissions: Questions and Answers – Draft Guidance for Industry and Food and Drug Administration Staff 08/23/13 10/16/18
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
basic practices used in the laboratory. A manual is given to all newcomers; it includes all the A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices.
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
OECD Principles of Good Laboratory Practice OECD.org – OECD
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
This guideline was developed to help protect clinical trial participants and patients receiving marketed products from potential adverse effects of pharmaceuticals, while avoiding unnecessary use of animals and other resources.
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Good Laboratory Practice; Regulations and Guidelines; Regulatory Roadmap; Animal & Veterinary Product; D.I.G.I.T. Good Clinical Practice; Good Laboratory Practice . Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
basic practices used in the laboratory. A manual is given to all newcomers; it includes all the A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices.
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
This part prescribes good laboratory practices for conducting studies that support or are intended to support applications for research or marketing permits for
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
ICMS
Good Laboratory Practice Standards Inspection Manual
Good Laboratory Practice; Regulations and Guidelines; Regulatory Roadmap; Animal & Veterinary Product; D.I.G.I.T. Good Clinical Practice; Good Laboratory Practice . Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
This guideline was developed to help protect clinical trial participants and patients receiving marketed products from potential adverse effects of pharmaceuticals, while avoiding unnecessary use of animals and other resources.
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
Tutorial Labcompliance
The Applicability of Good Laboratory Practice in Premarket
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
Good Laboratory Practice; Regulations and Guidelines; Regulatory Roadmap; Animal & Veterinary Product; D.I.G.I.T. Good Clinical Practice; Good Laboratory Practice . Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
The document is a critical factor of the good laboratory Practice Documentation is the accepted method of recording information for future reference. The major documents that need to be provided are protocols, logbook for usage, maintenance and calibration of equipment there should be well established SOPs. The following are some of the information that is routinely recorded in a laboratory:
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
[Code of Federal Regulations] National Toxicology Program
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
—– GOOD LABORATORY PRACTICE STANDARDS INSPECTION MANUAL September 1993 Prepared by: Scientific Support Branch Laboratory Data Integrity Assurance Division Office of Compliance Monitoring (EN342W) Washington, DC 20460 (703) 308
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
GOOD LABORATORY PRACTICES 1) Keep the manufacturer’s product insert for the laboratory test in use and be sure it is available to the testing personnel.
Tutorial Labcompliance
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
This guideline was developed to help protect clinical trial participants and patients receiving marketed products from potential adverse effects of pharmaceuticals, while avoiding unnecessary use of animals and other resources.
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
Good laboratory practice guidelines pdf” Keyword Found
[Code of Federal Regulations] National Toxicology Program
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
Good Laboratory Practice Standards Inspection Manual
Good Laboratory Practice (GLP) European Commission
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
basic practices used in the laboratory. A manual is given to all newcomers; it includes all the A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices.
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
GOOD LABORATORY PRACTICES 1) Keep the manufacturer’s product insert for the laboratory test in use and be sure it is available to the testing personnel.
GOOD LABORATORY PRACTICE eu-pregovori.rs
Good Laboratory Practice Guidelines PDF PDF documents
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Good Laboratory Practice Guidelines PDF PDF documents
GOOD LABORATORY PRACTICE eu-pregovori.rs
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Revised guidelines for good practice in IVF laboratories
ICMS
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
Good Laboratory Practice Guidelines PDF PDF documents
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Good Laboratory Practices and the ISO 90012000 standards
Good laboratory practice guidelines pdf” Keyword Found
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
MMWR Good Laboratory Practices for Waived Testing Sites
GOOD LABORATORY PRACTICE eu-pregovori.rs
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
CAC/GL 40-1993 Page 1 of 36 Revision 2003. Amendment 2010. GUIDELINES ON GOOD LABORATORY PRACTICE IN PESTICIDE RESIDUE ANALYSIS CAC/GL 40-1993
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good Laboratory Practices and the ISO 90012000 standards
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation an d Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
This part prescribes good laboratory practices for conducting studies that support or are intended to support applications for research or marketing permits for
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
Basic Good Laboratory Practice Think Bone
Good Laboratory Practices Standards Compliance Monitoring
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
[Code of Federal Regulations] National Toxicology Program
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
GOOD LABORATORY PRACTICES Centers for Medicare
GOOD LABORATORY PRACTICE eu-pregovori.rs
GOOD LABORATORY PRACTICES 1) Keep the manufacturer’s product insert for the laboratory test in use and be sure it is available to the testing personnel.
Good Laboratory Practice (GLP) European Commission
Good Laboratory Practices ppt SlideShare
OECD Principles of Good Laboratory Practice OECD.org – OECD
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
Good Laboratory Practices ppt SlideShare
Good Laboratory Practices and the ISO 90012000 standards
Good laboratory practice guidelines pdf” Keyword Found
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Good Laboratory Practice Standards Inspection Manual
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and
MMWR Good Laboratory Practices for Waived Testing Sites
Good Laboratory Practice Guidelines PDF PDF documents
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
OECD Principles of Good Laboratory Practice OECD.org – OECD
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
Revised guidelines for good practice in IVF laboratories
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Tutorial Labcompliance
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
OECD Principles of Good Laboratory Practice OECD.org – OECD
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good Laboratory Practice (GLP) European Commission
GOOD LABORATORY PRACTICE eu-pregovori.rs
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
MMWR Good Laboratory Practices for Waived Testing Sites
The Applicability of Good Laboratory Practice in Premarket Device Submissions: Questions and Answers – Draft Guidance for Industry and Food and Drug Administration Staff 08/23/13 10/16/18
ICMS
GOOD LABORATORY PRACTICES Centers for Medicare
Good Laboratory Practice Guidelines PDF PDF documents
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Tutorial Labcompliance
OECD Principles of Good Laboratory Practice OECD.org – OECD
GOOD LABORATORY PRACTICE eu-pregovori.rs
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Good laboratory practice guidelines pdf” Keyword Found
Good Laboratory Practice (GLP) European Commission
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Good Laboratory Practices and the ISO 90012000 standards
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
GOOD LABORATORY PRACTICE eu-pregovori.rs
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
Good Laboratory Practice Standards Inspection Manual
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
Good Laboratory Practice Guidelines PDF PDF documents
Revised guidelines for good practice in IVF laboratories
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
ICMS
Good Laboratory Practices and the ISO 90012000 standards
ESHRE Guideline Group on good practice in IVF labs December 2015. Revised guidelines for good practice in IVF laboratories (2015) Guideline of the European Society of Human Reproduction and Embryology. 2 . Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as ‘ESHRE’) developed the current clinical practice guideline, to provide clinical
Good Laboratory Practices and the ISO 90012000 standards
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
Revised guidelines for good practice in IVF laboratories
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
Revised guidelines for good practice in IVF laboratories
Good Laboratory Practice Standards Inspection Manual
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
Revised guidelines for good practice in IVF laboratories
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good laboratory practice training manual for the trainer: a tool for training and promoting good laboratory practice (GLP) concepts in disease endemic countries – 2nd ed. 1.Laboratories – organization and administration. 2.Laboratories – handbooks. 3.Laboratories techniques and proce-
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good Laboratory Practice (GLP). Guidelines for the Archiving of Electronic Raw Data in a GLP Environment† Working Group on Information Technology (AGIT)*
[Code of Federal Regulations] National Toxicology Program
Good Laboratory Practices and the ISO 90012000 standards
Revised guidelines for good practice in IVF laboratories
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
Tutorial Labcompliance
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
Good Clinical Laboratory Practice Canary Ltd
Tutorial Labcompliance
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
[Code of Federal Regulations] National Toxicology Program
GOOD LABORATORY PRACTICE eu-pregovori.rs
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Good Clinical Laboratory Practice Canary Ltd
Good Laboratory Practice (GLP) European Commission
OECD Principles of Good Laboratory Practice OECD.org – OECD
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
Good Clinical Laboratory Practice Canary Ltd
The Applicability of Good Laboratory Practice in Premarket
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
MMWR Good Laboratory Practices for Waived Testing Sites
Good laboratory practice guidelines pdf” Keyword Found
Revised guidelines for good practice in IVF laboratories
—– GOOD LABORATORY PRACTICE STANDARDS INSPECTION MANUAL September 1993 Prepared by: Scientific Support Branch Laboratory Data Integrity Assurance Division Office of Compliance Monitoring (EN342W) Washington, DC 20460 (703) 308
Good laboratory practice guidelines pdf” Keyword Found
Good Clinical Laboratory Practice Canary Ltd
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
This part prescribes good laboratory practices for conducting studies that support or are intended to support applications for research or marketing permits for
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
[Code of Federal Regulations] National Toxicology Program
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
OECD Principles of Good Laboratory Practice OECD.org – OECD
Good Laboratory Practice (GLP) European Commission
• Good Laboratory Practice Guidelines (“Official Gazette of the Republic of Serbia” No. 28/08), comply with directives 2004/9/EC and 2004/10 / EC. • Rulebook on the entry, content of the application and the costs of entry in the Register of laboratories that perform laboratory testing (“Official Gazette of the Republic of Serbia” No. 4/11) • Rulebook on the Good Laboratoy Practice
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Good Laboratory Practices and the ISO 90012000 standards
OECD Principles of Good Laboratory Practice OECD.org – OECD
GOOD LABORATORY PRACTICES 1) Keep the manufacturer’s product insert for the laboratory test in use and be sure it is available to the testing personnel.
GOOD LABORATORY PRACTICE eu-pregovori.rs
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
Revised guidelines for good practice in IVF laboratories
OECD Principles of Good Laboratory Practice OECD.org – OECD
ICMS
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Good Laboratory Practices and the ISO 90012000 standards
Good Laboratory Practice (GLP) European Commission
• A guideline is a consensus recommendation for best practice and should be used if a higher standard of practice is appropriate, particularly when setting up or modifying a laboratory test, or when contamination problems have occurred — guidelines are prefaced with a ‘G’
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Good Laboratory Practice Standards Inspection Manual
Tutorial Labcompliance
Revised guidelines for good practice in IVF laboratories
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
Good Laboratory Practices Standards Compliance Monitoring
Good Laboratory Practice (GLP) European Commission
Good Laboratory Practices ppt SlideShare
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
Good Laboratory Practices and the ISO 90012000 standards
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
Good Laboratory Practices Standards Compliance Monitoring
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Basic Good Laboratory Practice Think Bone
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
National Good Clinical Practice and Other Guidelines 124 Acknowledgements 125 iii. Preamble Clinical research is necessary to establish the safety and effective- ness of specifi c health and medical products and practices. Much of what is known today about the safety and effi cacy of specifi c prod-ucts and treatments has come from randomized controlled clinical trials1 that are designed
Basic Good Laboratory Practice Think Bone
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety OECD principles of Good Laboratory Practice shall be accepted in other member countries for purposes of assessment and other uses relating to the protection of man and the environment”. At a meeting in 1983, concerning the mutual recognition of compliance with GLP, the …
Good Laboratory Practices and the ISO 90012000 standards
Good Laboratory Practices ppt SlideShare
GOOD LABORATORY PRACTICES Centers for Medicare
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Basic Good Laboratory Practice Think Bone
GOOD LABORATORY PRACTICE eu-pregovori.rs
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Revised guidelines for good practice in IVF laboratories
Tutorial Labcompliance
The GLP regulations have reached this next stage of evolved understanding. The requirements are clear, the guidelines and interpretations are available, and the conflicts
Good Clinical Laboratory Practice Canary Ltd
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
GOOD LABORATORY PRACTICES Centers for Medicare
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
GOOD LABORATORY PRACTICE eu-pregovori.rs
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
[Code of Federal Regulations] National Toxicology Program
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
OECD Principles of Good Laboratory Practice OECD.org – OECD
GOOD LABORATORY PRACTICES Centers for Medicare
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
Good Clinical Laboratory Practice Canary Ltd
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
[Code of Federal Regulations] National Toxicology Program
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
Good laboratory practice guidelines pdf” Keyword Found
Good Laboratory Practice Guidelines PDF PDF documents
Chapter 3 – Code of Practice, Ethical Considerations and Legal Issues (42.71 KB) Chapter 4 – Sample Consent Forms (29.90 KB) Guidelines for Good Clinical Laboratory Practices (2.02 MB) Dietry. Ethics. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB
GOOD LABORATORY PRACTICES Centers for Medicare
GOOD LABORATORY PRACTICE eu-pregovori.rs
Good Laboratory Practices ppt SlideShare
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Basic Good Laboratory Practice Think Bone
ICMS
Good laboratory practice guidelines pdf” Keyword Found
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
Good Laboratory Practice (GLP) European Commission
ICMS
The Applicability of Good Laboratory Practice in Premarket Device Submissions: Questions and Answers – Draft Guidance for Industry and Food and Drug Administration Staff 08/23/13 10/16/18
Good Laboratory Practices Standards Compliance Monitoring
Good Laboratory Practice Guidelines PDF PDF documents
Good Laboratory Practices and the ISO 90012000 standards
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
Good Laboratory Practices ppt SlideShare
Good laboratory practice guidelines pdf” Keyword Found
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Good laboratory practice guidelines pdf” Keyword Found
GOOD LABORATORY PRACTICE eu-pregovori.rs
Good Laboratory Practices and the ISO 90012000 standards
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Tutorial Labcompliance
Good Laboratory Practices and the ISO 90012000 standards
Revised guidelines for good practice in IVF laboratories
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
Good Laboratory Practice Standards Inspection Manual
Good Laboratory Practices and the ISO 90012000 standards
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
GOOD LABORATORY PRACTICES Centers for Medicare
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
Basic Good Laboratory Practice Think Bone
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Tutorial Labcompliance
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
Good Laboratory Practice Guidelines PDF PDF documents
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
GOOD LABORATORY PRACTICE eu-pregovori.rs
Good Laboratory Practice Standards Inspection Manual
Basic Good Laboratory Practice Think Bone
The facility used in the processing of A-AS ’s should operate under Good Laboratory Practice (GLP) procedures, i.e., be kept in a clean, sanitary, and orderly manner, to prevent the introduction, transmission, or spread of communicable disease or the introduction of infectious
Basic Good Laboratory Practice Think Bone
Think Bone Consulting © 2009 Goals •Outline the concept of Good Laboratory Practice (GLP) •Provide some specific guidelines applicable to day to day work in the
Good laboratory practice guidelines pdf” Keyword Found
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Good Laboratory Practice Guidelines PDF PDF documents
The OECD Principles of Good Laboratory Practice is used as a regulatory control mechanism to assure the quality and integrity of non- clinical health and …
OECD Principles of Good Laboratory Practice OECD.org – OECD
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good laboratory practice guidelines pdf” Keyword Found
Good Clinical Practice (GCP) in Australia The Declaration of Helsinki was responsive to the revelations of the Nuremberg trials conducted after World War II, and its drafters sought to ensure that human subjects involved in clinical research would, in future, have their rights, safety and well-being placed above all other considerations in clinical research.
Good Laboratory Practices ppt SlideShare
The Applicability of Good Laboratory Practice in Premarket
Good laboratory practice guidelines pdf” Keyword Found
Good Laboratory Practice; Regulations and Guidelines; Regulatory Roadmap; Animal & Veterinary Product; D.I.G.I.T. Good Clinical Practice; Good Laboratory Practice . Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good
Good Laboratory Practices Standards Compliance Monitoring
What are ‘Best Laboratory Practices’ in Microbiology? Well, they are a way of developing control or, in analytical terms, having a ‘system suitability’ of the laboratory.
Good Laboratory Practices Standards Compliance Monitoring
[Code of Federal Regulations] National Toxicology Program
—– GOOD LABORATORY PRACTICE STANDARDS INSPECTION MANUAL September 1993 Prepared by: Scientific Support Branch Laboratory Data Integrity Assurance Division Office of Compliance Monitoring (EN342W) Washington, DC 20460 (703) 308
Basic Good Laboratory Practice Think Bone
The document is a critical factor of the good laboratory Practice Documentation is the accepted method of recording information for future reference. The major documents that need to be provided are protocols, logbook for usage, maintenance and calibration of equipment there should be well established SOPs. The following are some of the information that is routinely recorded in a laboratory:
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
Good Laboratory Practices ppt SlideShare
Revised guidelines for good practice in IVF laboratories
OECD Principles of Good Laboratory Practice OECD.org – OECD
GUIDELINES FOR POINT OF CARE TESTING (First Edition 2015) ii Guidelines for Point of Care Testing Print ISBN: 978-1-76007-002-1 indicate Guidelines or recommendations where compliance would be expected for good laboratory practice. 6 Guidelines for Point of Care Testing A Commentary is provided to give clarification to the Standards as well as to provide examples and …
Good Laboratory Practices ppt SlideShare
Basic Good Laboratory Practice Think Bone
[Code of Federal Regulations] National Toxicology Program
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
Good laboratory practice guidelines pdf” Keyword Found
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
ICMS
The document is a critical factor of the good laboratory Practice Documentation is the accepted method of recording information for future reference. The major documents that need to be provided are protocols, logbook for usage, maintenance and calibration of equipment there should be well established SOPs. The following are some of the information that is routinely recorded in a laboratory:
GOOD LABORATORY PRACTICE eu-pregovori.rs
Guideline developed within the Standing Committee on Plant Health with regard to the acceptability of data, whether or not performed in accordance with the principles of Good Laboratory Practice (GLP).
The Applicability of Good Laboratory Practice in Premarket
MMWR Good Laboratory Practices for Waived Testing Sites
Good Laboratory Practice Guidelines PDF PDF documents
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
Basic Good Laboratory Practice Think Bone
Good Laboratory Practice (GLP) deals with the organization, process and conditions under which laboratory studies are planned, performed, monitored, recorded and reported. GLP practices are intended to promote the quality and validity of test data.
GOOD LABORATORY PRACTICES Centers for Medicare
“Good Laboratory Practice –Mentions Good QC Laboratory Practice, stilipulates dilddetailed control measures • Recent Guideline from MHRA on Data Integrity essentially deals with GLP 05-June-2015 RRNANDAN@GMAIL.COM 11. Good Laboratory Practice Compliance req irementsrequirements • WHO GMP Guidelines –TRS 957 Annex 1 –WHO Good Practices for Pharmaceutical QC …
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
MMWR Good Laboratory Practices for Waived Testing Sites
The Applicability of Good Laboratory Practice in Premarket
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
GOOD LABORATORY PRACTICE eu-pregovori.rs
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
MMWR Good Laboratory Practices for Waived Testing Sites
[Code of Federal Regulations] National Toxicology Program
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
Good Laboratory Practice (GLP) European Commission
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good laboratory practice guidelines pdf” Keyword Found
Good Laboratory Practice Guidelines PDF PDF documents
The interaction between Good Laboratory Practices (GLP) and Quality Management Systems is not conflicting or exclusive but rather synergic -regardless of the specific environment in which they are applied-, since the general requirements of QMS Quality Systems lead to the compliance with the specific requirements of GLP. The purpose of this paper is to combine the specific requirements of
ICMS
OECD Principles of Good Laboratory Practice OECD.org – OECD
GLP: GOOD LABORATORY PRACTICE • GLP is an FDA regulation. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978. • GLP is a formal regulation that was created by the FDA (United states food and drug administration) in 1978.
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
Good Laboratory Practices and the ISO 90012000 standards
Revised guidelines for good practice in IVF laboratories
To bridge the gap between GCP and GLP, Good Clinical Laboratory Practice (GCLP) guidelines have been developed by a number of organisations (including the British Association of Research Quality Assurance (now the Research Quality Association),
GOOD LABORATORY PRACTICES Centers for Medicare
Good Laboratory Practice Standards Inspection Manual
The Principles of Good Laboratory Practice of the Organisation for Economic Cooperation and Development (OECD) form the basis of this series of guidance documents. This is the second version of the WHO Handbook on GLP. It is the result of experience gained since the first version was published. It also refers to material related to GLP devel- opments over the last seven years. Since the
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
MMWR Good Laboratory Practices for Waived Testing Sites
Home / Laboratory / Good Laboratory Practice (QMS ISO 17025:2017) Print ISO 17025 is a Quality Management System that can be applied by testing and calibration laboratories to ensure that laboratory testing is planned, performed, monitored, recorded and reported in an organised and controlled manner.
Good Laboratory Practice Standards Inspection Manual
Good Laboratory Practice (GLP) is concerned with the organisational process and the conditions under which laboratory studies are planned, performed, monitored, recorded, and reported.
GOOD LABORATORY PRACTICES Centers for Medicare
ICMS
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Basic Good Laboratory Practice Think Bone
Good Laboratory Practices and the ISO 90012000 standards
Good laboratory practice guidelines pdf keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on the this website
The Applicability of Good Laboratory Practice in Premarket
Good Laboratory Practices ppt SlideShare
MMWR Good Laboratory Practices for Waived Testing Sites
Adherence to Good Laboratory Practice Laboratory notebooks can be used to demonstrate compliance with appropriate health and safety regulations and conformity with other good practice guidelines.
Good Clinical Laboratory Practice Canary Ltd
Daids guidelines for good clinical laboratory practice standards the development of these gclp standards was a collaborative effort between ppd and the division of…
OECD Principles of Good Laboratory Practice OECD.org – OECD
EPA’s Good Laboratory Practice Standards (GLPS) compliance monitoring program ensures the quality and integrity of test data submitted to the Agency in support of a pesticide product registration under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), section 5 of the Toxic Substances Control Act (TSCA), and pursuant to testing
Revised guidelines for good practice in IVF laboratories
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
GOOD LABORATORY PRACTICE eu-pregovori.rs
The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations. The principles have been created in the context of harmonising testing …
Good Laboratory Practice Guidelines PDF PDF documents
Good laboratory practice guidelines pdf” Keyword Found
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
Malaysia Guideline for Good Clinical Practice, 3rd Ed Page 1 FOREWORD TO THE THIRD EDITION It has been more than a decade since the publication of the First Edition of the Malaysian Good Clinical Practice Guideline. Since then we have seen the publication of the Second Edition and this latest (Third) Edition marks another important milestone for clinical research in Malaysia. Over the last
SAFETY MANUAL OF GOOD LABORATORY PRACTICES
Good laboratory practice guidelines pdf” Keyword Found
GOOD LABORATORY PRACTICES Centers for Medicare
“Good Laboratory Practice GLPMA Expectations When Using a Contract Quality Assurance Service”, reviewed January 2015 Guidance on the use of GLP Study Report Amendments (april 2015) Guidance on test types stated on GLP compliance statements, reviewed January 2015
Good Laboratory Practices and the ISO 90012000 standards
Good Laboratory Practice (QMS ISO 17025) 1 Day Training
Good Laboratory Practice Standards Inspection Manual
guidelines is the regulation of GLP through the Principles of Good Laboratory Practice of the Organisation of Economic Cooperation and Development (OECD), since these have been discussed by an international panel of experts and have been agreed on at an international
Good Laboratory Practices Standards Compliance Monitoring
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org
MMWR Good Laboratory Practices for Waived Testing Sites
Laboratory Practice; ensure that after termination of the study, the study plan, the final report, raw data and supporting material are transferred to the archives.
Good Laboratory Practices ppt SlideShare
The guideline was developed with consideration of the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries and the World Health Organization (WHO).
Basic Good Laboratory Practice Think Bone
GUIDELINES ON GOOD LABORATORY PRACTICE IN fao.org