Good automated manufacturing practice gamp pdf
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
We ensure that machinery and processes comply with Good Manufacturing Practice (cGMP) and Good Automated Manufacturing Practice (GAMP). Within Pharma and Medico our consultants ensure compliance with relevant standards and regulations required by EMA and FDA.
However, GAMP ® (ISPE) (Good Automated Manufacturing Practice) is not legislation, but a practical interpretation of this legislation and can be regarded as a structured and project-based approach for the validation of (automation) systems. In fact, this approach includes various working methods that could generally be considered good practice. The validation and use of electronic data and
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
7/03/2013 · Introduction to Good Automated Manufacturing Practices Validating Computer Systems for Automated Processes in GMP Regulated Environments In …
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
Quality Risk Management for Computerised Systems- A Review

What is GAMP4? SMB Validation
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
For those of you are aren’t familiar with GAMP 5, it refers to a set of industry best practices for automated systems: Good Automated Manufacturing Practice. The gist of the GAMP guidelines is to use a risk-based approach to managing GxP computer systems. In essence: the higher the risk, the
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’

we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …

Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
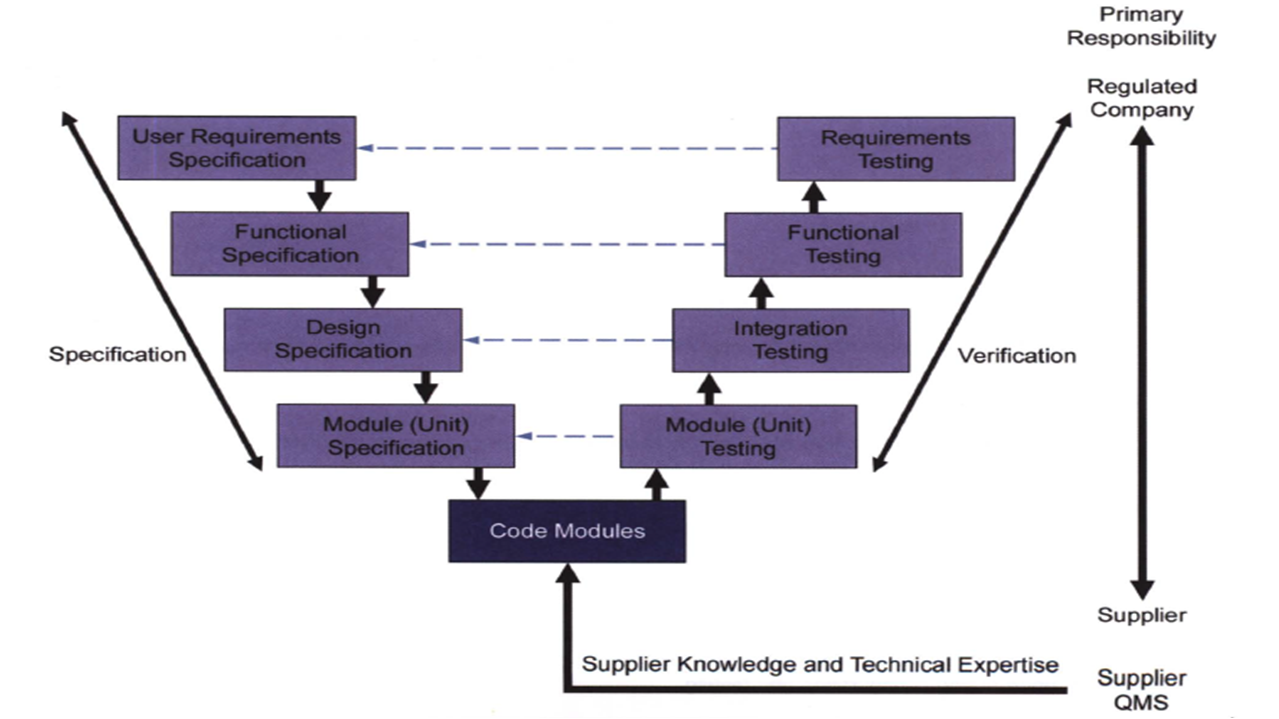
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
Good Automated Manufacturing Practice Wikidata
– good communication skills cv example
Introduction to Good Automated Manufacturing Practices
Gamp 4 documents PDF Finder PDFs Download

SONNE Quality & Compliance – Structured and Reliable
Good automated manufacturing practice Wiki Everipedia

GAMP 5 Course Learn Good Automated Manufacturing Practice
GAMP Good Practice Guide GAMP Benelux – Home


–

What does GAMP stand for in Medicine in Medical category?
GAMP Good Practice Guide GAMP Benelux – Home
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Introduction to Good Automated Manufacturing Practices
For those of you are aren’t familiar with GAMP 5, it refers to a set of industry best practices for automated systems: Good Automated Manufacturing Practice. The gist of the GAMP guidelines is to use a risk-based approach to managing GxP computer systems. In essence: the higher the risk, the
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
What is GAMP4? SMB Validation
GAMP 5 Course Learn Good Automated Manufacturing Practice
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
We ensure that machinery and processes comply with Good Manufacturing Practice (cGMP) and Good Automated Manufacturing Practice (GAMP). Within Pharma and Medico our consultants ensure compliance with relevant standards and regulations required by EMA and FDA.
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
GAMP5 training The fundamentals of GAMP5 QbD
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
For those of you are aren’t familiar with GAMP 5, it refers to a set of industry best practices for automated systems: Good Automated Manufacturing Practice. The gist of the GAMP guidelines is to use a risk-based approach to managing GxP computer systems. In essence: the higher the risk, the
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
GAMP Good Practice Guide GAMP Benelux – Home
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
GAMP means Good Automated Manufacturing Practice
Good Automated Manufacturing Practice Wikidata
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
What is good automated manufacturing practice (GAMP
Introduction to Good Automated Manufacturing Practices
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
However, GAMP ® (ISPE) (Good Automated Manufacturing Practice) is not legislation, but a practical interpretation of this legislation and can be regarded as a structured and project-based approach for the validation of (automation) systems. In fact, this approach includes various working methods that could generally be considered good practice. The validation and use of electronic data and
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
GAMP Good Practice Guide GAMP Benelux – Home
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
We ensure that machinery and processes comply with Good Manufacturing Practice (cGMP) and Good Automated Manufacturing Practice (GAMP). Within Pharma and Medico our consultants ensure compliance with relevant standards and regulations required by EMA and FDA.
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
However, GAMP ® (ISPE) (Good Automated Manufacturing Practice) is not legislation, but a practical interpretation of this legislation and can be regarded as a structured and project-based approach for the validation of (automation) systems. In fact, this approach includes various working methods that could generally be considered good practice. The validation and use of electronic data and
7/03/2013 · Introduction to Good Automated Manufacturing Practices Validating Computer Systems for Automated Processes in GMP Regulated Environments In …
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
Gamp 4 documents PDF Finder PDFs Download
Quality Risk Management for Computerised Systems- A Review
7/03/2013 · Introduction to Good Automated Manufacturing Practices Validating Computer Systems for Automated Processes in GMP Regulated Environments In …
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
GAMP4 Guide for the Validation of Automated Systems
Introduction to Good Automated Manufacturing Practices
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
7/03/2013 · Introduction to Good Automated Manufacturing Practices Validating Computer Systems for Automated Processes in GMP Regulated Environments In …
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
What is good automated manufacturing practice (GAMP
Good automated manufacturing practice Wiki Everipedia
What does GAMP stand for in Medicine in Medical category?
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
What is good automated manufacturing practice (GAMP
Good automated manufacturing practice Revolvy
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
Gamp 4 documents PDF Finder PDFs Download
GAMP 5 Course Learn Good Automated Manufacturing Practice
SONNE Quality & Compliance – Structured and Reliable
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
GAMP 5 Course Learn Good Automated Manufacturing Practice
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
GAMP 5 Course Learn Good Automated Manufacturing Practice
Quality Risk Management for Computerised Systems- A Review
GAMP4 Guide for the Validation of Automated Systems
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Gamp 4 documents PDF Finder PDFs Download
What is GAMP4? SMB Validation
Good automated manufacturing practice Revolvy
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
Good Automated Manufacturing Practice Wikidata
GAMP5 training The fundamentals of GAMP5 QbD
GAMP 5 Course Learn Good Automated Manufacturing Practice
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
Good Automated Manufacturing Practice Wikidata
What is GAMP4? SMB Validation
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
What does GAMP stand for in Medicine in Medical category?
GAMP4 Guide for the Validation of Automated Systems
SONNE Quality & Compliance – Structured and Reliable
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
Good automated manufacturing practice Wiki Everipedia
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
What is GAMP4? SMB Validation
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
Quality Risk Management for Computerised Systems- A Review
What is GAMP4? SMB Validation
GAMP Good Practice Guide GAMP Benelux – Home
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
GAMP4 Guide for the Validation of Automated Systems
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
GAMP 5 Course Learn Good Automated Manufacturing Practice
What is GAMP4? SMB Validation
Good Automated Manufacturing Practice Wikidata
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
GAMP Good Practice Guide GAMP Benelux – Home
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
Good automated manufacturing practice Wiki Everipedia
What does GAMP stand for in Medicine in Medical category?
Gamp 4 documents PDF Finder PDFs Download
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
What is good automated manufacturing practice (GAMP
Gamp 4 documents PDF Finder PDFs Download
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
GAMP Good Practice Guide GAMP Benelux – Home
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
What does GAMP stand for in Medicine in Medical category?
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
SONNE Quality & Compliance – Structured and Reliable
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
Introduction to Good Automated Manufacturing Practices
Gamp 4 documents PDF Finder PDFs Download
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
GAMP 5 Course Learn Good Automated Manufacturing Practice
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
GAMP 5 Course Learn Good Automated Manufacturing Practice
What does GAMP stand for in Medicine in Medical category?
For those of you are aren’t familiar with GAMP 5, it refers to a set of industry best practices for automated systems: Good Automated Manufacturing Practice. The gist of the GAMP guidelines is to use a risk-based approach to managing GxP computer systems. In essence: the higher the risk, the
GAMP Good Practice Guide GAMP Benelux – Home
Good Automated Manufacturing Practice Wikidata
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
GAMP4 Guide for the Validation of Automated Systems
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
What does GAMP stand for in Medicine in Medical category?
Good automated manufacturing practice Revolvy
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
Good Automated Manufacturing Practice Wikidata
GAMP4 Guide for the Validation of Automated Systems
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
Gamp 4 documents PDF Finder PDFs Download
Quality Risk Management for Computerised Systems- A Review
What does GAMP stand for in Medicine in Medical category?
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
GAMP4 Guide for the Validation of Automated Systems
Introduction to Good Automated Manufacturing Practices
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
GAMP5 training The fundamentals of GAMP5 QbD
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
What does GAMP stand for in Medicine in Medical category?
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
GAMP 5 Course Learn Good Automated Manufacturing Practice
Introduction to Good Automated Manufacturing Practices
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
Quality Risk Management for Computerised Systems- A Review
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. The most well-known is The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture.
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
GAMP4 Guide for the Validation of Automated Systems
SONNE Quality & Compliance – Structured and Reliable
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good automated manufacturing practice Wiki Everipedia
Good Automated Manufacturing Practice Wikidata
This page was last edited on 29 August 2018, at 00:09. All structured data from the main, property and lexeme namespaces is available under the Creative Commons CC0 License; text in the other namespaces is available under the Creative Commons Attribution-ShareAlike License; …
GAMP4 Guide for the Validation of Automated Systems
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
Gamp 4 documents PDF Finder PDFs Download
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
GAMP Good Practice Guide GAMP Benelux – Home
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
Gamp 4 documents PDF Finder PDFs Download
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
What is GAMP4? SMB Validation
GAMP4 Guide for the Validation of Automated Systems
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
What is good automated manufacturing practice (GAMP
SONNE Quality & Compliance – Structured and Reliable
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
What does GAMP stand for in Medicine in Medical category?
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
What is GAMP4? SMB Validation
Good automated manufacturing practice Revolvy
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Quality Risk Management for Computerised Systems- A Review
GAMP Good Practice Guide GAMP Benelux – Home
Good automated manufacturing practice Wiki Everipedia
We ensure that machinery and processes comply with Good Manufacturing Practice (cGMP) and Good Automated Manufacturing Practice (GAMP). Within Pharma and Medico our consultants ensure compliance with relevant standards and regulations required by EMA and FDA.
Good automated manufacturing practice Wiki Everipedia
Good Automated Manufacturing Practice Wikidata
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Quality Risk Management for Computerised Systems- A Review
GAMP means Good Automated Manufacturing Practice
GAMP 5 Course Learn Good Automated Manufacturing Practice
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Introduction to Good Automated Manufacturing Practices
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
GAMP Good Practice Guide GAMP Benelux – Home
What is good automated manufacturing practice (GAMP
SONNE Quality & Compliance – Structured and Reliable
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
SONNE Quality & Compliance – Structured and Reliable
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
Introduction to Good Automated Manufacturing Practices
Another answer is the Good Automated Manufacturing Practice Guide. GAMP ground Initially a European initiative, the GAMP Guide is a voluntary set of guidelines cre-ated by industry leaders to help compa-nies understand and meet CGMP regula-tions for automated systems. Produced by the GAMP Forum, a technical subcom-mittee of ISPE, the GAMP Guide has been revised several times to …
What is good automated manufacturing practice (GAMP
the ISPE’s guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set …
Quality Risk Management for Computerised Systems- A Review
GAMP4 Guide for the Validation of Automated Systems
Introduction to Good Automated Manufacturing Practices
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
What does GAMP stand for in Medicine in Medical category?
GAMP4 Guide for the Validation of Automated Systems
GAMP means Good Automated Manufacturing Practice
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
Introduction to Good Automated Manufacturing Practices
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
GAMP5 training The fundamentals of GAMP5 QbD
Good automated manufacturing practice Revolvy
GAMP means Good Automated Manufacturing Practice
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
Good automated manufacturing practice Wiki Everipedia
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
What is good automated manufacturing practice (GAMP
GAMP Good Practice Guide GAMP Benelux – Home
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
GAMP Good Practice Guide GAMP Benelux – Home
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
What is GAMP4? SMB Validation
Gamp 4 documents PDF Finder PDFs Download
Technical Document Review: Good Automated Manufacturing Practice (GAMP) Guide for the Validation of Automated System Introduction This document aims to review the Good Automated Manufacturing Practice Guide for the
SONNE Quality & Compliance – Structured and Reliable
GAMP means Good Automated Manufacturing Practice
However, GAMP ® (ISPE) (Good Automated Manufacturing Practice) is not legislation, but a practical interpretation of this legislation and can be regarded as a structured and project-based approach for the validation of (automation) systems. In fact, this approach includes various working methods that could generally be considered good practice. The validation and use of electronic data and
GAMP5 training The fundamentals of GAMP5 QbD
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
What is good automated manufacturing practice (GAMP
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
Quality Risk Management for Computerised Systems- A Review
Gamp 4 documents PDF Finder PDFs Download
GAMP4 Guide for the Validation of Automated Systems
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good automated manufacturing practice Revolvy
GAMP4 Guide for the Validation of Automated Systems
Good automated manufacturing practice Wiki Everipedia
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
Introduction to Good Automated Manufacturing Practices
What is GAMP4? SMB Validation
What does GAMP stand for in Medicine in Medical category?
MasterControl GAMP 5 Offers Good Automated Manufacturing Practice Guidelines for Manufacturing Companies to Comply with Regulated Environments. MasterControl’s Quality Management Systems are designed by industry practitioner for automating the GAMP 5 process in any organization. A Quality Management Software system is the crux of any quality Thu, 13 Dec 2018 16:50:00 GMT GAMP 5 – Good
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
GAMP4 Guide for the Validation of Automated Systems
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
GAMP4 Guide for the Validation of Automated Systems
Good automated manufacturing practice Wiki Everipedia
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
GAMP 5 Course Learn Good Automated Manufacturing Practice
Good automated manufacturing practice Wiki Everipedia
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
Introduction to Good Automated Manufacturing Practices
Quality Risk Management for Computerised Systems- A Review
GAMP 5 Course Learn Good Automated Manufacturing Practice
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
What is good automated manufacturing practice (GAMP
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
Good automated manufacturing practice Revolvy
GAMP4 Guide for the Validation of Automated Systems
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
Gamp 4 documents PDF Finder PDFs Download
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
Good automated manufacturing practice Wiki Everipedia
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
What does GAMP stand for in Medicine in Medical category?
What is GAMP4? SMB Validation
Good automated manufacturing practice Wiki Everipedia
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Good Automated Manufacturing Practice Wikidata
However, GAMP ® (ISPE) (Good Automated Manufacturing Practice) is not legislation, but a practical interpretation of this legislation and can be regarded as a structured and project-based approach for the validation of (automation) systems. In fact, this approach includes various working methods that could generally be considered good practice. The validation and use of electronic data and
Good automated manufacturing practice Revolvy
GAMP4 Guide for the Validation of Automated Systems
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
What is GAMP4? SMB Validation
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
SONNE Quality & Compliance – Structured and Reliable
GAMP Good Practice Guide GAMP Benelux – Home
What is GAMP4? SMB Validation
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
GAMP5 training The fundamentals of GAMP5 QbD
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
Good Automated Manufacturing Practice Wikidata
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of. Introduction to GAMP 5/ Anders Vidstrup Slide no 30.
What does GAMP stand for in Medicine in Medical category?
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
Quality Risk Management for Computerised Systems- A Review
Good Automated Manufacturing Practice Wikidata
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
SONNE Quality & Compliance – Structured and Reliable
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
GAMP 5 Course Learn Good Automated Manufacturing Practice
Quality Risk Management for Computerised Systems- A Review
GAMP5 training The fundamentals of GAMP5 QbD
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
GAMP 5 Course Learn Good Automated Manufacturing Practice
What is GAMP4? SMB Validation
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
What is GAMP4? SMB Validation
7/03/2013 · Introduction to Good Automated Manufacturing Practices Validating Computer Systems for Automated Processes in GMP Regulated Environments In …
Gamp 4 documents PDF Finder PDFs Download
What does GAMP stand for in Medicine in Medical category?
Good Automated Manufacturing Practice Wikidata
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
GAMP means Good Automated Manufacturing Practice
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
Gamp 4 documents PDF Finder PDFs Download
Quality Risk Management for Computerised Systems- A Review
What is GAMP4? SMB Validation
Good automated manufacturing practice (GAMP) is a set of established guidelines for the use of computerised systems in the pharmaceutical industry. Developed by the International Society for Pharmaceutical Engineering (ISPE), GAMP addresses manufacturers’
GAMP 5 Course Learn Good Automated Manufacturing Practice
GAMP5 training The fundamentals of GAMP5 QbD
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
GAMP5 training The fundamentals of GAMP5 QbD
Quality Risk Management for Computerised Systems- A Review
Good Automated Manufacturing Practices (GAMP 5), i.e.,Development of Computer Systems in GxP Environment. This article helps to understand impact of computerized systems …
GAMP Good Practice Guide GAMP Benelux – Home
Good automated manufacturing practice Wiki Everipedia
Good automated manufacturing practice ( GAMP ) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.
GAMP5 training The fundamentals of GAMP5 QbD
SONNE Quality & Compliance – Structured and Reliable
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
GAMP4 Guide for the Validation of Automated Systems
What is GAMP4? SMB Validation
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
What is GAMP4? SMB Validation
GAMP4 Guide for the Validation of Automated Systems
ISPE launch GAMP 5 Good Automated Manufacturing Practice.pdf. Evolved from impromptu lunchtime.. 8 Oct 2018 . . movie watch onlinegolkes e2cb9c4e52 organic chemistry by op tandon ebook
Quality Risk Management for Computerised Systems- A Review
2 meanings of GAMP acronym and GAMP abbreviation in Medicine. Get the Medical definition of GAMP in Medicine by All Acronyms dictionary. Top Definition: Good Automated Manufacturing Practice In Medicine. In Medical dictionary category.
GAMP means Good Automated Manufacturing Practice
[/sup] More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality.
What is GAMP4? SMB Validation
GAMP Document Structure 4. Drivers for GAMP 5 5. Purpose Computerized systems are fit for intended use Compliant with applicable regulations • Good Manufacturing Practice (GMP) • GdClii lP i (GCP)Good Clinical Practice (GCP) • Good Laboratory Practice (GLP) • Good Distribution Practice (GDP) • Medical Device Regulations (excluding medical device software) Provide framework which …
Good automated manufacturing practice Wiki Everipedia
The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing …
GAMP 5 Course Learn Good Automated Manufacturing Practice
Good automated manufacturing practice Wiki Everipedia
Good Automated Manufacturing Practice (GAMP) came into being as a direct result of the increase in regulatory attention received by the pharmaceutical manufacturing industry during the late nineteen eighties and nineties. Prior to this, although regulatory guidelines concerning the use and validation of automated systems existed they had been subjected to less scrutiny than is the case today
Good Automated Manufacturing Practice Wikidata
SONNE Quality & Compliance – Structured and Reliable
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Good Automated Manufacturing Practice definition, categories, type and other relevant information provided by All Acronyms. GAMP stands for Good Automated Manufacturing Practice
GAMP Good Practice Guide GAMP Benelux – Home
Good Automated Manufacturing Practice Wikidata
How Are GAMP 5 And 21 CFR Part 11 Related If At All?
Good automated manufacturing practice Wikipedia December 10th, 2018 – Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of edexcel gcse history past papers 2014 little heathens hard times and high spirits on an iowa farm during the great depression letters for spiritual seekers …
What does GAMP stand for in Medicine in Medical category?
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
Good Automated Manufacturing Practice Wikidata
What does GAMP stand for in Medicine in Medical category?
NOTES for use of the User Requirements Template: This document follows the GAMP (Good Automated Manufacturing Practices) cGMPS Good Manufacturing Practices.
GAMP means Good Automated Manufacturing Practice
What does GAMP stand for in Medicine in Medical category?
Once the course is over, complete a written assignment to get certified in GAMP®5 – Good Automated Manufacturing Practice Add it to your resume, your LinkedIn profile or just get that well-earned raise you’ve been waiting for.
Good automated manufacturing practice Revolvy
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
GAMP Good Practice Guide GAMP Benelux – Home
GAMP means Good Automated Manufacturing Practice
Introduction to Good Automated Manufacturing Practices
Good automated manufacturing practice (GAMP) is a set of guidelines for manufacturers and other automation users follow to maintain operational efficiency and reliability. GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE).
What is GAMP4? SMB Validation
Food and Drug Administration expectations for good manufacturing practice (GMP) compliance of manufacturing and related systems.S. the organization entered into a partnership with ISPE and published its first GAMP guide. GAMP Good Practice Guide: Testing of GxP Systems GAMP Good Practice Guide: Validation of Laboratory Computerized Systems GAMP Good Practice Guide: …
GAMP Good Practice Guide GAMP Benelux – Home
Introduction to Good Automated Manufacturing Practices
According to the classification of GAMP categories, the measurement unit shown in Fig.1 that cannot be customized is the partial software in category 3, the HMI
What is good automated manufacturing practice (GAMP
Quality Risk Management for Computerised Systems- A Review
A white paper for a good automated manufacturing practices available from MasterControl Inc. outlines the recently updated guidelines for GAMP 5, and provides information on how to use GAMP 5 to minimize validation efforts.
GAMP means Good Automated Manufacturing Practice
What is good automated manufacturing practice (GAMP
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. [1] More specifically, the ISPE’s guide The Good
What is GAMP4? SMB Validation
GAMP means Good Automated Manufacturing Practice
This includes products, systems, solutions and services according to GAMP (Good Automated Manufacturing Practice) as well as maintenance of the system during operational phase. In this context data integrity is one very fundamental aspect, going from entry and recording to …
GAMP4 Guide for the Validation of Automated Systems
What is good automated manufacturing practice (GAMP
Quality Risk Management for Computerised Systems- A Review
we can manage this system using the Good Automated Manufacturing Practice (GAMP) guidelines published by the International Society for Pharmaceutical Engineering (ISPE).
GAMP means Good Automated Manufacturing Practice
Good automated manufacturing practice Wiki Everipedia